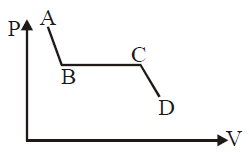
Solution:
If the portion AB is slant, it indicates that both pressure (P) and volume (V) are changing. In thermodynamic terms, this suggests that AB could represent a compression or expansion, process where both pressure decreases and volume decreases.
Given that you mentioned that the correct answer is "the liquid state of matter," it is likely that AB represents the compression of a liquid.
In many thermodynamic diagrams, when a liquid is compressed or expands slightly, both its pressure and volume can change, though not as dramatically as in gases. This phase corresponds to the liquid region of a substance's phase diagram, where both pressure and volume are decreasing, which is why AB represents the liquid state.
To summarize:
- AB is slant: Both pressure and volume are decreasing.
- Liquid state: Liquids are not perfectly incompressible; hence, both pressure and volume can change, but the changes in volume are relatively small compared to gases.
This slant line indicates the behaviour of a liquid as it is compressed, leading to the conclusion that AB represents the liquid state of matter.
Leave a Reply